EXPERTISE
Concept to Production
At Resolution Medical our expertise is wrapped in deep anatomical experience, underpinned by a distinct understanding of how to seamlessly transition from concept to our formal phase gate development process to production.
From the initial spark of design concepts to manufacturing execution, we’re deeply committed to ensuring that your medical device is of high quality, repeatable, and competitive in your market.
Areas of Expertise
Click below to see examples of our experience
| Active Implantable Programs | System |
|---|---|
| Peripheral Nerve System | IPG/Lead |
| Tibial Nerve System | IPG/Lead |
| Heart Monitoring System | IPG |
| Novel Cardiac Pacing System | Lead |
| Heart Monitoring System | IPG |
| Inter-atrial Shunt with Electronics | Implant/Delivery System |
| Neuromodulation System (ENT) | IPG/Header/Lead |
| Neuromodulation System (DBS) | IPG/Lead |
| Percutaneous LVAD (long-term) | Pump/Driveline/Implants |
| Percutaneous LVAD (short-term) | Pump/Driveline |
| Percutaneous LVAD (short-term) | Pump/Driveline |
| Percutaneous RVAD | Pump/Driveline |
| Total Artificial Heart | Pump/Driveline |
| LVAD (long-term) | IPG/Header |
| Pulmonary Hypertension System | Implantable Can & Anchor |
| Novel Cardiac Pacing System | Lead |
| Implantable Port w/Electronics | IPG |
| Structural Heart Programs | System |
|---|---|
| Transcatheter Aortic Valve Replacement | Delivery System |
| Transcatheter Pulmonary Valve Replacement | Delivery System |
| Transcatheter Mitral Valve Replacement | Delivery System |
| Transcatheter Mitral Valve Repair (DMR) | Implant/Delivery System |
| Transcatheter Mitral Valve Repair (DMR/FMR) | Delivery System |
| Transapical & Transcatheter Mitral Valve Repair (FMR) | Implant/Delivery System |
| Transcatheter Tricuspid Valve Repair | Implant/Delivery System |
| Transcatheter Tricuspid Valve Replacement | Implant/Delivery System |
| Percutaneous LVAD | Implant/Delivery System |
| Percutaneous RVAD | Implant/Delivery System |
| Inter-atrial Shunt | Delivery System |
| Inter-atrial Shunt | Implant |
| Pulmonary Hypertension | Implant/Delivery System |
| LAA | Delivery System |
| PFO Closure | Implant/Delivery System |
| Embolic Protection | Implant/Delivery System |
| Structural Heart Accessory System | Delivery System |
| Neuromodulation Programs | System |
|---|---|
| Peripheral Nerve System | IPG/Delivery/Externals |
| Peripheral Nerve System | Lead/Delivery/Externals |
| Tibial Nerve System | IPG/Lead/Delivery |
| Neuromodulation System (ENT) | IPG/Lead |
| Neuromodulation System (ENT) | Lead |
| Neuromodulation System (DBS) | IPG |
| Neuromodulation System (DBS) | Lead |
| Neuromodulation System (SCS) | IPG |
| Neuromodulation System (SCS) | Header |
| Neuromodulation System (SCS) | Lead |
| Lead Implant Kit | Delivery system |
| Cardiology & Vascular Programs | System |
|---|---|
| Neurovascular Stent Delivery System | Implant/Delivery System |
| Peripheral Intravascular Ultrasound (IVUS) | Delivery System |
| Optical Coherence Tomography (OCT) | Delivery System |
| Pulmonary Biopsy System | Delivery System |
| Transapical Mitral Valve Repair | Implant/Delivery System |
| Tricuspid Valve Repair – Surgical | Implant/Delivery System |
| Aortic Disease Tissue Repair | Implant/Delivery System |
| Heart Monitoring System | Implant |
| LAA | Delivery System |
| PFO Closure | Implant/Delivery System |
| Embolic Protection System | Implant/Delivery System |
| Valved Introducer Kit | Delivery System |
| Thrombectomy System | Delivery System |
| Electrophysiology Programs | System |
|---|---|
| Pulsed Field Ablation System | Delivery System |
| RF Ablation System | Delivery System |
| Heart Monitoring System | IPG |
| Esophageal Ablation Protection System | Delivery System |
| Cardiac Pacing System | Lead/Delivery |
| Temporary Pacing Lead | Lead/Delivery |
| Heart Monitoring Device | IPG |
| Heart Monitoring Device | IPG |
| Cryo Ablation System | Delivery System |
| RF Ablation System | Delivery System |
| Heart Failure Programs | System |
|---|---|
| Percutaneous LVAD (long-term) | Implant/Delivery System |
| Percutaneous RVAD | Implant/Delivery System |
| Percutaneous LVAD (short-term) | Implant/Delivery System |
| Total Artificial Heart | Implant |
| Total Artificial Heart | Driveline |
| Inter-atrial Shunt | Implant |
| Inter-atrial Shunt | Delivery System |
Left Ventricular Biopsy System | Delivery System |
Transcatheter Aortic Valve Replacement | Delivery System |
Transcatheter Pulmonary Valve Replacement | Delivery System |
Transcatheter Mitral Valve Replacement | Delivery System |
Transcatheter Mitral Valve Repair (DMR) | Implant/Delivery System |
Transcatheter Mitral Valve Repair (DMR/FMR) | Delivery System |
Transapical & Transcatheter Mitral Valve Repair (FMR) | Implant/Delivery System |
Transcatheter Tricuspid Valve Repair | Implant/Delivery System |
Transcatheter Tricuspid Valve Replacement | Implant/Delivery System |
| Additional Markets | System |
|---|---|
| Peripheral Vascular – Atherectomy | Delivery System |
| Endoscopy – GI | Delivery System |
| ENT – Cochlear | Implant/Delivery |
| ENT – Cochlear | Consumable/Capital |
| ENT – Cochlear | Lead |
| Urology – Prostate | Implant/Delivery |
| Urology – Prostate | Delivery System |
| Oncology – Pancreatic | Implant/Delivery |
| Oncology – Pulmonary | Delivery System |
| Robotics | Delivery System |
Your Guide Through the
Full Product Lifecycle

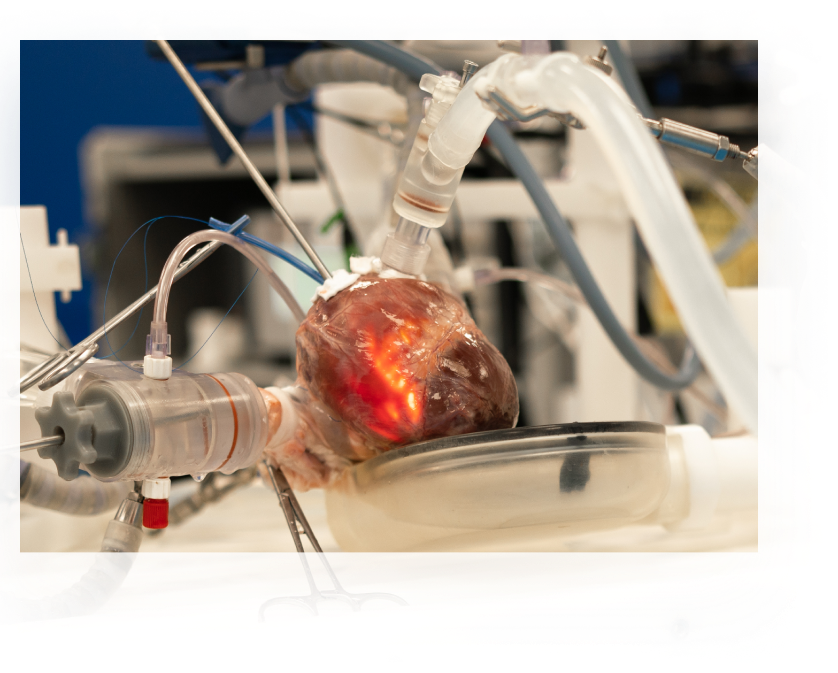
Our team becomes your team, and we partner throughout the entire product lifecycle – from conceptualization to manufacturing and beyond.
With our formal phase gate process, we ensure transparency and direction at every juncture. Need to navigate regulatory milestones or secure funding approvals? We’re by your side (even co-locating if needed), guiding every step of the way. Our experience allows us to sidestep potential challenges and anticipate obstacles in advance, ensuring a smoother path and clearer destination.
NPI Process Development: Structured Product Transfer
Select the transfer structure that best fits your needs. Each pathway is designed to accommodate distinct timelines and objectives, ensuring your transfer is customized and highly effective.
“Same as Same”
Achieve consistency with the existing setup by transferring all components, suppliers, and documentation without a hitch, ensuring minimal disruption.
Process Improvements
Refinements and supplier enhancements optimize cost and quality, leading to a larger return on investment. Focus on process development without impacting design.
Design and Process Improvements
Delve into process improvements and design modifications, resulting in the most comprehensive transformation with the potential for significant returns.
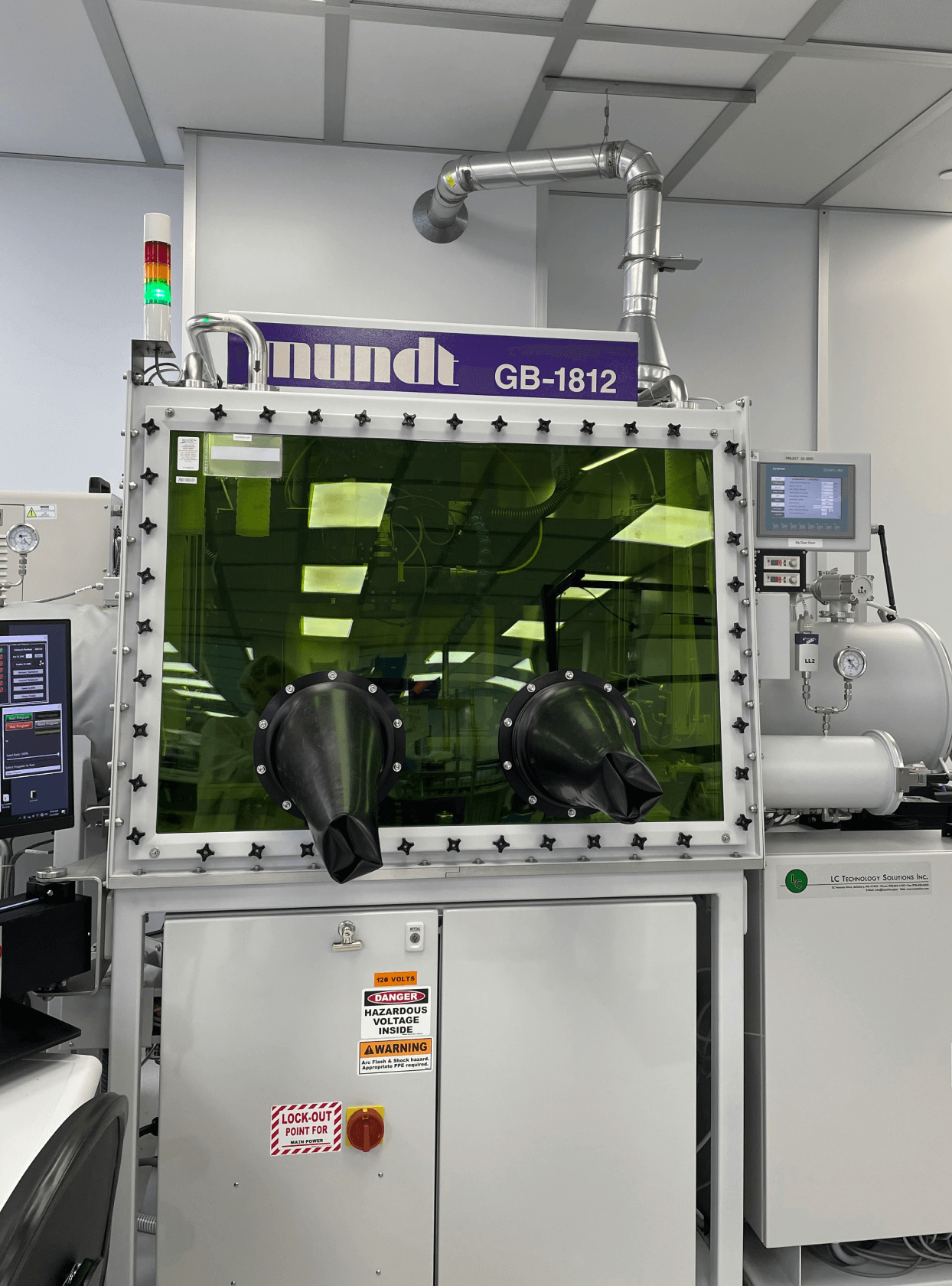


Manufacturing Expertise
At Resolution Medical, we have a 36,000 square foot facility dedicated to manufacturing, FDA registration, and decades of experience. Our team is well-prepared to accommodate a wide range of manufacturing needs from low to high volume, and our collaborative process emphasizes transparency and open communication to ensure the success.
We have multiple ISO Class 7 Cleanrooms providing a controlled environment for precise finished goods assembly, sterile barrier packaging, product labeling, inspections, and cleaning. We also offer a UL certified electrostatic discharge area, non-clean room manufacturing, and a distribution center designed for efficient logistics, all aimed at assisting you in the most effective way possible.
Click through each of our capabilities to learn more:
- Metals (Nitinol, Titanium, Stainless, Platinum)
- Plastics (Peek, Nylons, etc.)
- Sub-assemblies
- Testing and analysis
- Production Glovebox for hermetic welds
- Laser Marking
- Vertical and horizontal presses
- Insert Molding (hubs, tips, handles)
- Complements Additive Manufacturing
- TPE, TPU, PEEK, Nylons, Pebax, HDPE, Polycarbonate, etc.
- Quick change tooling platform designed to lower tooling costs and reduce lead-times on smaller part geometries
- Multiple Carbon M2 printers
- Formlabs and Stratasys printers
- Biocompatible and sterilizable materials
- Compliments injection molding
- Optimizes component complexity through quick-turn iterations
- Low to mid-volume production efficiencies
Manufacturing Expertise
At Resolution Medical, we have a 36,000 square foot facility dedicated to manufacturing, FDA registration, and decades of experience. Our team is well-prepared to accommodate a wide range of manufacturing needs from low to high volume, and our collaborative process emphasizes transparency and open communication to ensure the success.
We have multiple ISO Class 7 Cleanrooms providing a controlled environment for precise finished goods assembly, sterile barrier packaging, product labeling, inspections, and cleaning. We also offer a UL certified electrostatic discharge area, non-clean room manufacturing, and a distribution center designed for efficient logistics, all aimed at assisting you in the most effective way possible.
Laser Cutting & Welding
Utilize expertise in metals, plastics, sub-assemblies, testing, welds, and laser marking for complete production solutions.
- Metals (Nitinol, Titanium, Stainless, Platinum)
- Plastics (Peek, Nylons, etc.)
- Sub-assemblies
- Testing and analysis
- Production Glovebox for hermetic welds
- Laser Marking

Molding
Leverage vertical/horizontal presses, insert molding, quick-change tooling, and additive manufacturing for cost-effective, fast production.
- Vertical and horizontal presses
- Insert Molding (hubs, tips, handles)
- Complements Additive Manufacturing
- TPE, TPU, PEEK, Nylons, Pebax, HDPE, Polycarbonate, etc.
- Quick change tooling platform designed to lower tooling costs and reduce lead-times on smaller part geometries

Additive Manufacturing
Use Carbon M2, Formlabs, and Stratasys printers with biocompatible materials for complex, optimized production.
- Multiple Carbon M2 printers
- Formlabs and Stratasys printers
- Biocompatible and sterilizable materials
- Compliments injection molding
- Optimizes component complexity through quick-turn iterations
- Low to mid-volume production efficiencies

Nitinol System Development
Nitinol’s ability to undergo and excessive deformations and return to an intended shape and function (superelasticity) has made it a critical material in trans-catheter medical devices. At Resolution Medical we not only understand Nitinol and how to process it, but we know how to design with this unique material to meet your clinical needs and incorporate it into any catheter-based system you can dream up. Our engineers have extensive experience in concept generation, prototyping, process development, part integration, regulatory submissions and clinical analysis that was born out of working with startup companies. This breath of experience allows us to be better members of your design team and mitigate hurdles in your development projects well in advance.
Collaborative Nitinol development
If you already have a design and are looking for a partner to refine your design with and get it ready for your next stage of clinical development, we are here for that too. Everyone approaches this material differently and we enjoy seeing where you came from and helping you get to where you want to go.
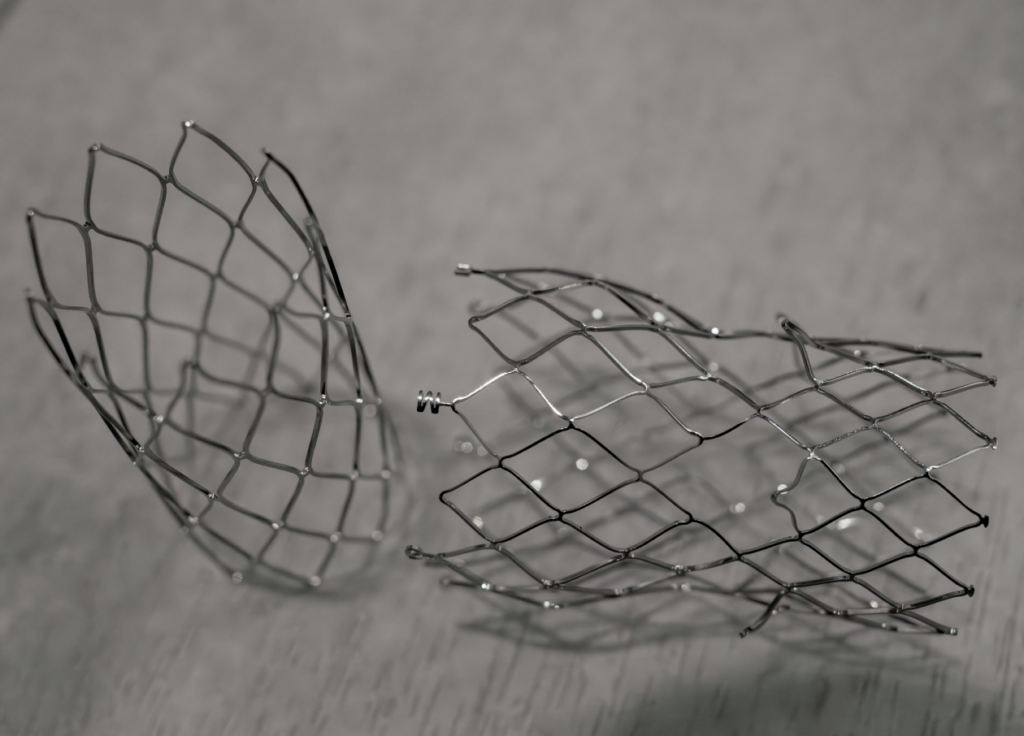

Comprehensive Capabilities of Nitinol Components and Device
With a deep understanding of Nitinol’s unique properties and behavior under various processes, our team expertly controls the design output and fine-tunes component performance. Our comprehensive capabilities allow us to rapidly respond to test results and iterate designs, ensuring optimal results at every stage.
Design: Our experienced team excels in designing Nitinol components, optimizing their geometry to ensure the device’s critical functionality is achieved.
Laser Cutting: We offer in-house femto and fiber laser cutting for both tube and flat sheet materials, providing precise and efficient cutting solutions.
Shape Setting: Our NiTi team specializes in fixture design to shape Nitinol parts accurately. The subsequent heat treatment permanently sets the desired shape, ensuring precision in every component.
Electropolishing: Our electropolishing process smooths Nitinol surfaces, enhancing corrosion resistance, fatigue resilience, and biocompatibility for medical applications.
Passivation: We offer passivation to Nitinol components to form a layer of biocompatible Titanium oxide layer.
Testing Capabilities:
- Contact Af Test
- Non-contact Af Test
- Coupon Tensile Test
- Radial Force Test
- Bench Testing of the whole device
- BioSim Test


Quality is a personal pledge for us.
Our experts, processes, equipment, and services are dedicated to ensuring compliance, safety, and precision every step of the way.

